The Antidote to Erase Clean in Place Process Blind Spots
The line’s running hot. Then—bam—your clean-in-place cycle misfires. Foam lingers. Valves don’t close. The rinse never clears. You stare at a screen, hoping the numbers stabilize before QA flags another batch.
Clean-in-place process failures aren’t random. They’re predictable. And preventable. With the right CIP automation strategy, you can eliminate blind spots, restore stability, and keep your plant running safely and compliantly—24/7.
This Verdusco Automation article explores why CIP fails, how smarter control systems fix it, and how you can take back control.
Why Clean-in-Place Process Fails in Manufacturing
The problem often tracks to aging infrastructure.
Here’s what’s really going on out on the floor:
Worn valves don’t seal perfectly, leading to inconsistent spray coverage and chemical cross-contamination.
Faulty sensors misread values while the actual product line languishes.
Outdated automation logic causes cycles to run blindly due to the lack of real-time sensor feedback.
The aftermath of something being off ripples through. You can sense it in a line that sounds hollow. Or in the frustration of watching thousands of gallons of water and expensive chemicals go straight down the drain. Or in the cold sweat of a failed audit.
These are the symptoms of your plant telling you it’s time for a change.
How Smarter Control Systems Bring Back CIP Stability
The antidote lies in modern automation. With the right redesign, CIP systems stop being unpredictable and start being dependable.
By integrating advanced PID loops and high-fidelity sensors, the smart CIP system monitors conditions in real time.
No more waiting on a timer. Smarter controllers (active 24/7) monitor and balance timers, flow, pressure, and temperature.
Autonomous detection of conductivity measurements confirms CIP fulfillment. Rinse cycles won’t be cut short. Steam purges won’t fail halfway.
Conditions and setpoints are maintained accordingly. Valves will snap shut at the exact right moment. Thermal kill steps are actually achieved. Steam will purge lines until every drop of residue is gone. Rinsed water is crystal clear.
These new features are safeguards against threats, compliance failures, and production downtime.
It’s smarter automation taking risks off the table.
Restoring Hygiene Compliance with Smart Automation
Consider two plants.
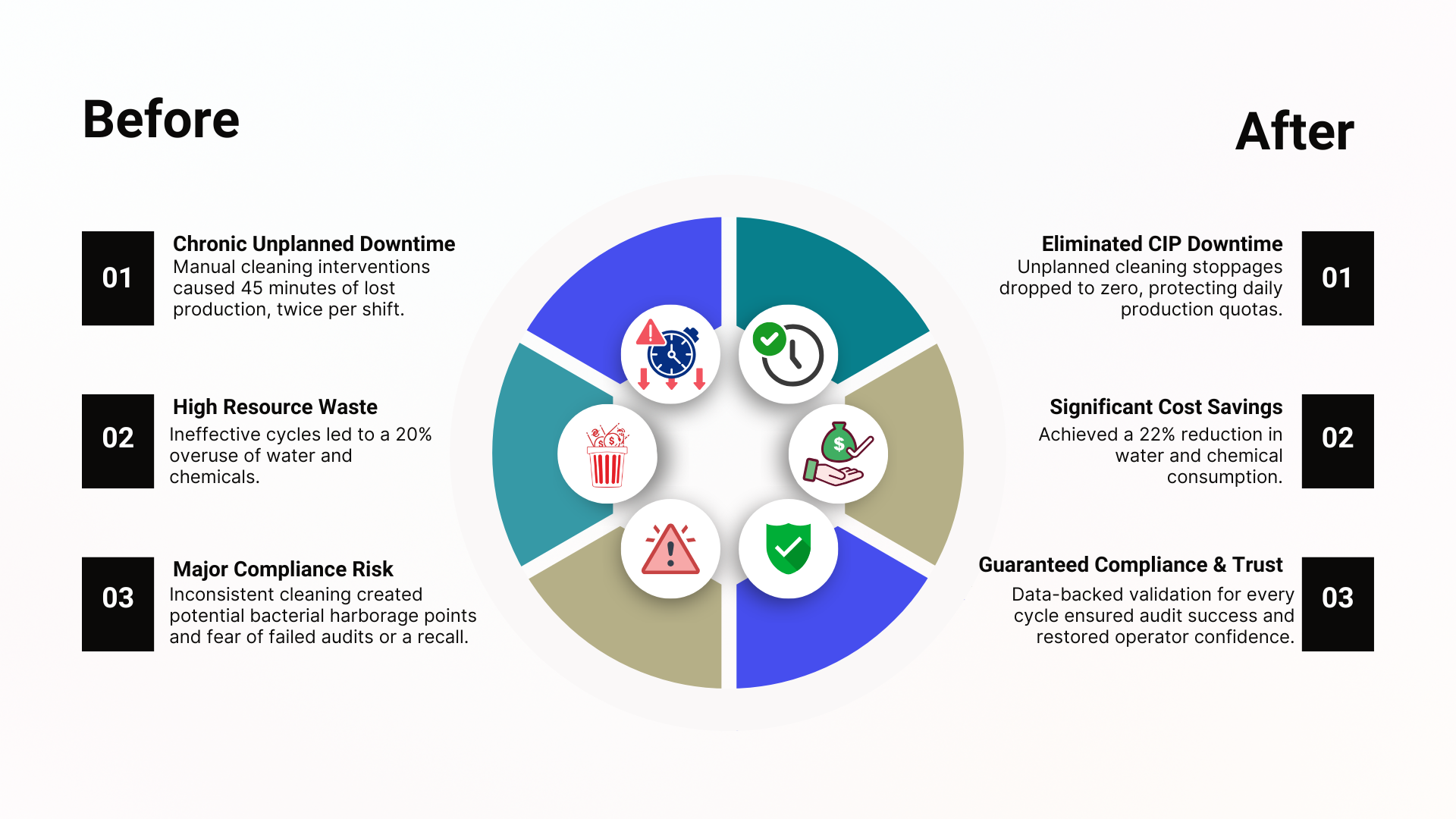
Regional dairy manufacturer of premium, pasteurized milk and cream
Their legacy CIP system for the filler machines was a chronic headache. The automation logic was timer-based, blind to actual conditions. Worn spray ball nozzles gave inconsistent coverage, while a failing temperature sensor routinely sent false readings. Operators would often discover residual film on equipment surfaces after a “completed” cycle, forcing a full, manual stop-and-scrub.
In a case like this, the transformation begins with a holistic CIP automation redesign:
Replacing failing temperature and conductivity sensors with high-accuracy, hygienic models.
Upgrading spray balls and swapping critical valves for assured-shutoff models.
Rewriting the PLC logic, shifting from simple timers to a state-based process that only advanced upon receiving verified sensor feedback. The system will now require a confirmed temperature kill step and a conductivity-proven rinse before allowing production to resume.
The changes would be striking:
Fig. 1. Potential Dairy CIP Automation Upgrade Before-After Results (Source: Verdusco Automation).
The difference would be night and day. No more guessing, no more wasted rinses, and no more sticky residue waiting to trigger a compliance failure.
Contract Pharmaceutical Manufacturer Producing Sterile Ophthalmic Solutions
Their CIP process for the product contact tanks was severely vulnerable. The system relied on manual verifications and basic automation, leaving a dangerous data gap. Worn diaphragm valves were prone to micro-leaks, and inconsistent flow meter readings made it impossible to guarantee full surface coverage. QA engineers had to verify cleaning efficacy with swab tests after every cycle. That process alone took more than 90 minutes and halted production. Without a digital, data-backed audit trail, compliance was constantly at risk, forcing unnecessarily long, chemical-heavy wash cycles just to stay safe.
Here, the CIP automation overhaul could include:
Installing high-precision sensors for temperature, conductivity, and turbidity.
Replacing all critical valves with hygienic, leak-proof models.
Calibrating flow meters to pharmaceutical standards.
Engineering new control logic that automates sequences and generates unalterable data logs. The system holds at each critical phase (pre-rinse, caustic wash, final rinse) until sensor data confirms all parameters are met (only then advancing to the next step).
Here’s a before-and-after recap:
Fig. 2. Potential Pharma CIP Automation Upgrade Before-After Results (Source: Verdusco Automation).
The lesson? CIP automation is not a patch. It’s a pivot. It takes plants from firefighting to stability, with fewer recalls, smoother operations, and happier inspectors.
Blueprint Your CIP Automation Makeover
The message is simple:
A clean-in-place process without blind spots isn’t a dream. It’s a decision. Manufacturers who embrace smarter automation not only stop compliance headaches but also give their teams peace of mind.
Verdusco Automation helps you get there with solutions designed for your scale and budget.
Our services include:
Control systems design and integration: custom-built for stability in cleaning and production loops.
Automation logic upgrades: modern PLC and SCADA updates that cut downtime and compliance risk.
Maintenance-friendly dashboards: real-time monitoring so operators and engineers can spot issues before they escalate.
You don’t need to settle for patch jobs. You can have a process that works right the first time, every time.
Contact Verdusco Automation today!
📩: https://www.verduscoautomation.com/contact
Note:
This article is a reference point for what smart CIP automation can achieve. Every upgrade must be tailored to your industry, niche, and process specifics to deliver real results.